shelf life calculator for pharmaceutical products
The absence of an expiration date on any drug product packaged after September 29 1979 except for those drugs specifically exempt by. Accelerated stability testing and shelf-life calculation.
Establishing the Shelf Life of Pharmaceutical Products.
. Shelf Life Calculation Of Drugs. Each batch is distinguished by its own shelf life which can be called the true batch shelf life. Shelf life calculator for pharmaceutical products Saturday June 4 2022 Edit.
The shelf life of a drug-device combination product is determined by the shortest estimated shelf life. Recognizing that calibrated estimate of shelf life is an estimate with uncertainty a lower interval estimate is obtained as a conservative estimate of shelf life. Also it is necessary to find the mark.
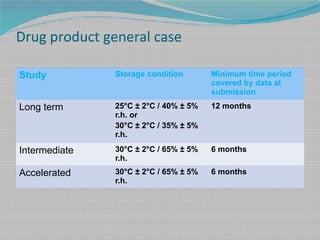
Arrhenius plot to get k at 25 ºC and from this we can calculate the shelf-. This article proposes new terminology that distinguishes between different concepts involved in the discussion of the shelf life of pharmaceutical products. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically.
Shelf life Pharmaceutical Stability Shelf Life August 1 2010 2 Definitions of Shelf Life ICH Q1E ICH Guideline Q1E defines shelf life as The shelf life of a pharmaceutical product is the maximum time at which the true mean response of a stability limiting characteristic crosses th e acceptance criterion. Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various. Expiry with date in days in months or in years.
A batch is a fixed quantity of product for example 100000 tablets. This guideline applies to human and veterinary medicines. Such comprehensive and common language is currently lacking from various guidelines which confuses implementation and impedes comparisons of different methodologies.
The batch is a single sample of the pharmaceutical products manufacturing process at. 32 days x 156. N 50-25 10 25.
The five new. For example the shelf life of a product at 50 C is 32 days. In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field.
The medical device industry in particular places a large emphasis on testing the shelf life of products. Shelf life or the expiration date of a product relative to its manufacture date when kept in the indicated environmental conditions is an essential aspect of the product lifecycle development for combination products but often lacks clarity. Input the TAA and TRT values both in Celsius for your product.
Absence of an Expiration Date. Shelf life is a product of physical microbiological and chemical processes triggered by any one of a multitude of contributing factors. The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date.
Thats because as time goes on a products Sterile Barrier System can become altered as it faces stresses from time and the ambient environment. This knowledge is critical across industries from consumer goods to pharmaceuticals. Normal storage temperature is 25 C.
The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life. By using quantile approach shelf life can be directly related to a Quality Standard. Suppose Q10 3.
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services. This guideline applies to human and veterinary medicines. At that time Q10n 3 25 156.
Guidelines on Stability Studies of Pharmaceutical Products and Shelf Life Estimation. At that time Q10n 3 25 156. This online service helps you to know how long your product is in good condition.
Shelf life calculator excel Thursday February 17 2022 Edit. After entering data you push. Note that using the 50 quantile ie mean is also possible but in contrast to current.
What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration date of a drug product and the stability testing needed to ensure that. Expected shelf life is usually.
The shelf-life of a drug product is the time that the average drug charac- teristic eg potency remains within an approved specification after manufacture. A pharmaceutical product is typically manufactured in batches. Calculate half-life and shelf life of pharmaceutical products and drugs.
Statistica Sinica 112001 737-745 DRUG SHELF-LIFE ESTIMATION Jun Shao and Shein-Chung Chow University of Wisconsin and StatPlus Inc. Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology. Batch and Product Shelf Life.

Specialty Pharmacy The Complete Pharmacist Specialty Pharmacy Pharmacy National Cancer Institute
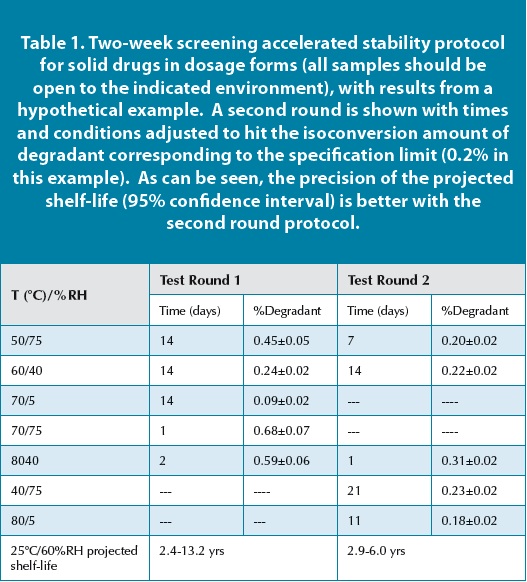
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services
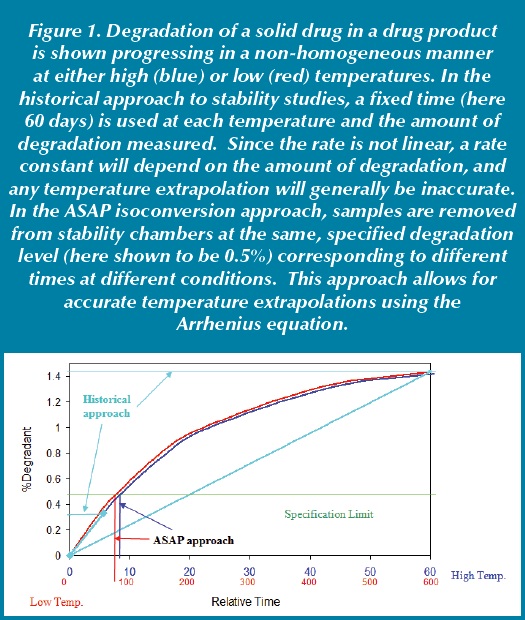
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube

Free Legacy Food Endure Probiotic By Legacy Premium 49 99 Http Www Freelegacyfood Com Endure Probiotic B Probiotics Freeze Drying Food Digestive Health

Stability Testing And Shelf Life Estimation

Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75 Super User
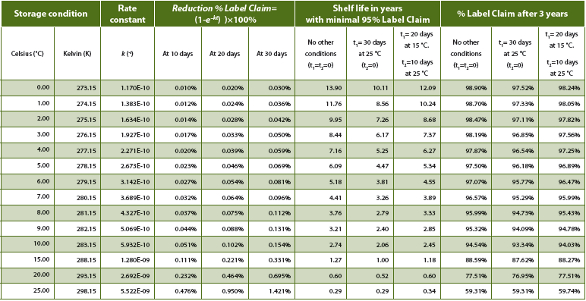
Establishing And Managing The Drug Product Stability Budget Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Checking And Calculating The Shelf Life Expiration Date Sap Help Portal

Shelf Life Calculation Of Drugs

Shelf Life Of Foods First Order Kinetics Example Youtube

Finding A Drug S Real Expiration Date Npr

Shelf Life Calculator For Composites And Other Materials

Analyzing Data From Stability Studies Youtube
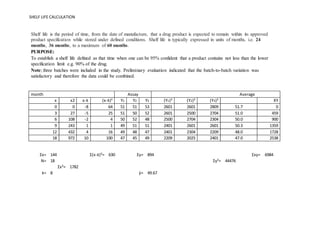
Shelf Life Calculation Of Drugs

Shelf Life Calculator For Composites And Other Materials

Shelf Life Calculator For Composites And Other Materials